ApiFix®
Designed to fill a crucial gap in the treatment of adolescent idiopathic scoliosis (AIS), the ApiFix procedure provides a viable alternative to both bracing and fusion for select patients.
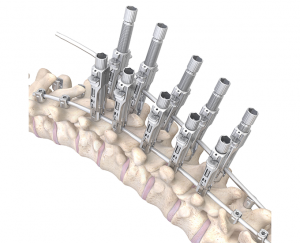
Less Invasive: With three pedicle screws and a familiar posterior approach, the unilateral technique is less invasive than spinal fusion and simpler than the other fusionless procedures requiring an anterior approach.
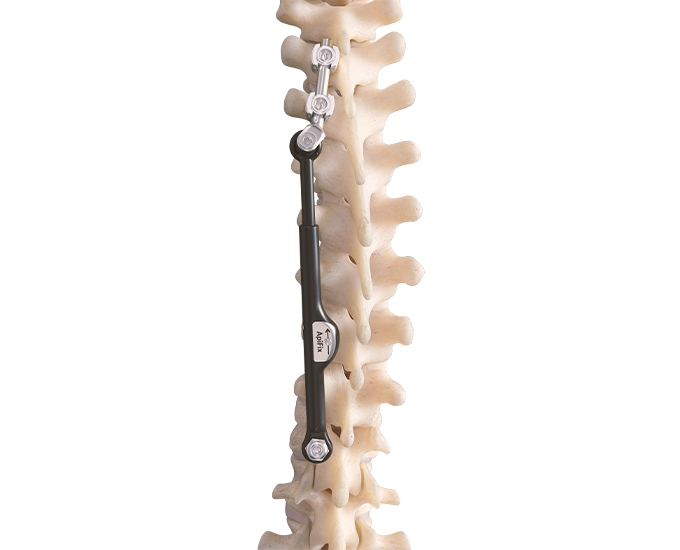
The unique ApiFix approach provides a viable alternative to failed bracing before considering spinal fusion for many patients as the least invasive spine deformity correction option. The MID-C system acts as an “internal brace” with motion-preserving polyaxial joints and a patented unidirectional, self-adjusting rod mechanism allowing additional post-operative correction over time and is ultimately removable.
Predictable Correction: The majority of curve correction is achieved during the index procedure. The self-adjusting rod allows for additional post-operative correction over time and can accommodate further growth.
Preserves Motion: The novel design of the ApiFix system, using polyaxial joints and a self-adjusting rod, allows a degree of motion that is not possible with rigid implants.
Long-Term Clinical Track Record: Since 2012, more than 800 AIS patients have been treated with the ApiFix procedure.
Visit www.ApiFix.com to learn more about this technology.