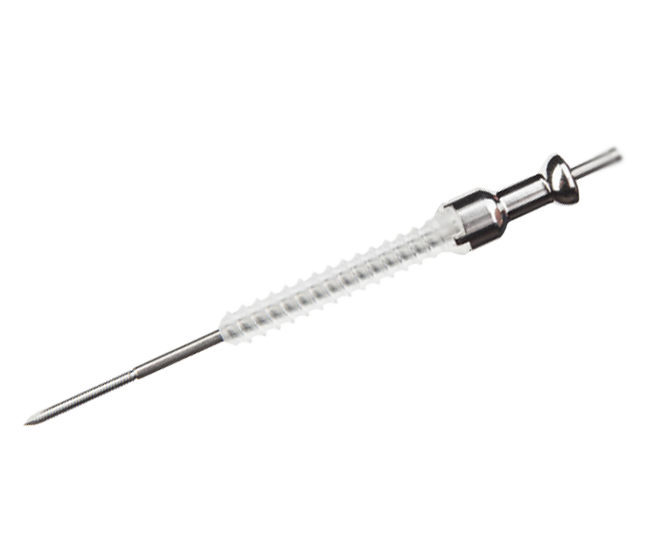
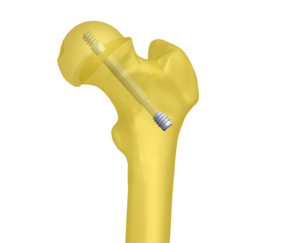
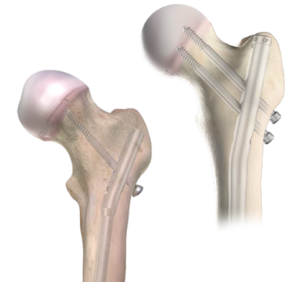
Bioretec Cannulated ActivaScrew
OrthoPediatrics distributes the Bioretec ActivaScrew Cannulated product in the USA at children’s hospitals. The next generation resorbable polymer (PLGA- self reinforced Poly-85L-lactic/15 glycolic acid) has an established safety profile with 400,000+ implants to date and helps eliminate the need for second surgery to remove implants. Implants maintain mechanical integrity for at least 8 weeks after implantation during fracture healing while bone gains strength.